bromine bohr model|3.6: Electron Arrangement : iloilo Set 12, 2023 — Learn how to draw the Bohr model of Bromine (Br) atom with four electron shells and 35 electrons. Follow the steps to find the number of protons, neutrons, and electrons in Bromine and place them in the shells according to .
Start streaming gay Pinoy porn at BoyfriendTV right now. Filipino guys are so sweet and they seem so innocent, but we know they have some of the horniest minds and kinkiest needs. Watch as these adorable guys from the Philippines tempt and tease you, showing off their solo pleasures from home on their cams and in homemade porn movies.This programme allows students to study a wide array of Home Economics and Business related modules. This programme is the first in its kind as it is the only programme that offers a BA (Hons) in Home .
PH0 · Lecture #3, Atomic Structure (Rutherford, Bohr Models)
PH1 · Khan Academy
PH2 · Bromine Bohr model
PH3 · Bromine Bohr Model
PH4 · Bromine (Br)
PH5 · Bromine
PH6 · Bohr Diagrams of Atoms and Ions
PH7 · Bohr
PH8 · 5.4: The Bohr Model of The Atom
PH9 · 3.6: Electron Arrangement
SNSU aims to produce quality graduates that respond to the dynamics of national and international standards. Its goals and objectives are focused on the 5-point agenda: Instruction, Research Innovation and Extension (RIE), Resources Generation, Policy Implementation and Good Governance.
bromine bohr model*******The bromine Bohr model has a nucleus with 35 protons and 45 neutrons. Surrounding this nucleus are four electron shells, holding a total of 35 electrons. To draw the bromine Bohr model, represent the 35 protons, 45 neutrons, and 35 electrons. Begin by sketching the nucleus, and then . Tingnan ang higit paBromine has 35 protons, 45 neutrons, and 35 electrons. Learn how to find: Bromine protons neutrons electrons Tingnan ang higit paThe nucleus of a bromine atom contains 35 protons and 45 neutrons. So draw the nucleus of bromine atom as follows: Now in the next step, draw the 1stelectron shell and start . Tingnan ang higit paThe 2nd electron shell (containing s subshell and p subshell) can hold up to a maximum of 8 electrons. So draw the 2ndelectron shell as follows: In the above image, 2 represents the 2nd electron shell that contains 2s and 2p subshells. And the green and . Tingnan ang higit pa
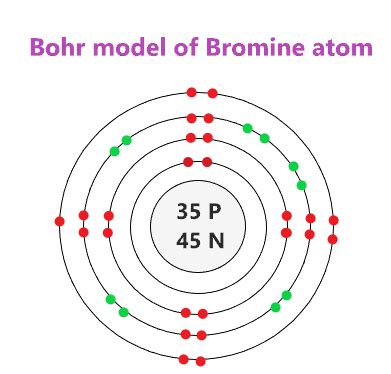
Remember that we have a total of 35 electrons. The 1st electron shell (containing s subshell) can hold up to a maximum of 2 electrons. So draw the 1stelectron shell as follows: In the above image, 1 represents the 1st electron shell that . Tingnan ang higit paSet 12, 2023 — Learn how to draw the Bohr model of Bromine (Br) atom with four electron shells and 35 electrons. Follow the steps to find the number of protons, neutrons, and electrons in Bromine and place them in the shells according to .Ene 30, 2023 — The Bohr model shows the atom as a central nucleus containing protons and neutrons with the electrons in circular orbitals at specific .Hul 28, 2020 — Learn how to draw the Bohr-Rutherford diagram for bromine, a halogen with 18 electrons in its third shell and 5 extra electrons in its fourth shell. Watch a video explanation by chemistNATE, a chemistry teacher and YouTuber.
Khanmigo is now free for all US educators! Plan lessons, develop exit tickets, and so much more with our AI teaching assistant.

Ago 10, 2022 — Define an energy level in terms of the Bohr model. Discuss how the Bohr model can be used to explain atomic spectra. Describe the arrangement of electrons using the shell .3.6: Electron ArrangementDis 13, 2023 — Bohr’s model of the hydrogen atom gave an exact explanation for its observed emission spectrum. The following are his key contributions to our understanding of atomic .bromine bohr modelSet 14, 2009 — Learn about the history and principles of atomic models, from Rutherford's alpha scattering to Bohr's quantum theory. See examples of hydrogen atom spectra, energy levels, .bromine bohr model 3.6: Electron ArrangementSet 14, 2009 — Learn about the history and principles of atomic models, from Rutherford's alpha scattering to Bohr's quantum theory. See examples of hydrogen atom spectra, energy levels, .
Learn about bromine, a halogen with symbol Br and atomic number 35, and its electron configuration in the Bohr model. See the history, properties, and identifiers of bromine.
Bohr’s Model of an Atom. The Bohr model describes the structure of an atom as a central nucleus containing protons and neutrons, with electrons orbiting in specific energy levels around it. Electrons can jump between these energy .Ago 20, 2024 — Bohr model, description of the structure of atoms proposed in 1913 by the Danish physicist Niels Bohr. The Bohr model of the atom, a radical departure from earlier, classical descriptions, was the first that incorporated .The Bohr model of the hydrogen atom (Z = 1) or a hydrogen-like ion (Z > 1), where the negatively charged electron confined to an atomic shell encircles a small, positively charged atomic nucleus and where an electron jumps between orbits, is accompanied by an emitted or absorbed amount of electromagnetic energy (hν). [1] The orbits in which the electron may travel are shown as .Abr 9, 2024 — The bromine electron configuration, denoted as [] 4s 2 3d 10 4p 5 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within the atom.This configuration can be determined through .
Khanmigo is now free for all US educators! Plan lessons, develop exit tickets, and so much more with our AI teaching assistant.
Name: Bromine Symbol: Br Atomic Number: 35 Atomic Mass: 79.904 amu Melting Point:-7.2 °C (265.95 K, 19.04 °F) Boiling Point: 58.78 °C (331.93 K, 137.804 °F) Number of Protons/Electrons: 35 Number of Neutrons: 45 Classification: Halogen Crystal Structure: Orthorhombic Density @ 293 K: 3.119 g/cm 3 Color: Red Atomic StructureHul 28, 2020 — Bromine has 18 electrons in its third shell because it is past Zinc on the periodic table. Then, you go back to the fourth shell and put 5 extra electrons in.
Ago 14, 2020 — Since Bohr’s model involved only a single electron, it could also be applied to the single electron ions He +, Li 2+, Be 3+, and so forth, which differ from hydrogen only in their nuclear charges, and so one-electron atoms and ions are collectively referred to as hydrogen-like atoms.The energy expression for hydrogen-like atoms is a generalization of the hydrogen .Hul 26, 2024 — In the bromine orbital diagram, the 1s subshell holds two electrons, the 2s subshell carries another pair, the 2p subshell encompasses six electrons, the 3s subshell contains two electrons, the 3p subshell carries six electrons, the 4s subshell holds two electrons, the 3d subshell carries ten electrons, and the 4p subshell accommodates five electrons, totaling thirty .Hul 27, 2022 — The Bohr model of the hydrogen atom explains the connection between the quantization of photons and the quantized emission from atoms. Bohr described the hydrogen atom in terms of an electron moving in a circular orbit about a nucleus. He postulated that the electron was restricted to certain orbits characterized by discrete energies.
which is identical to the Rydberg equation in which R ∞ = k h c. R ∞ = k h c. When Bohr calculated his theoretical value for the Rydberg constant, R ∞, R ∞, and compared it with the experimentally accepted value, he got excellent agreement. Since the Rydberg constant was one of the most precisely measured constants at that time, this level of agreement was astonishing .Many modifications have been introduced to the Bohr model, most notably the Sommerfeld model or Bohr – Sommerfeld model, which suggested that electrons move around a nucleus in elliptical orbits rather than circular orbits .Hul 12, 2013 — The most instantly recognizable image of an atom resembles a miniature solar system with the concentric electron paths forming the planetary orbits and the nucleus at the centre like the sun. In July of 1913, Danish .Despite the fact that the energies are essentially correct, the Bohr model masks the true quantum nature of the electron, which only emerges from a fully quantum mechanical analysis. Exercise 1.8.1 Calculate a value for the Bohr radius using Equation \(\ref{1.8.16}\) to check that this equation is consistent with the value 52.9 pm.
Ago 26, 2023 — Following the work of Ernest Rutherford and his colleagues in the early twentieth century, the picture of atoms consisting of tiny dense nuclei surrounded by lighter and even tinier electrons continually moving about the nucleus was well established. This picture was called the planetary model, since it pictured the atom as a miniature “solar system” with the electrons .The Bohr model represents the structure of an atom developed by Danish physicist Niels Bohr in 1913. According to this model, the atomic structure is similar to that of the solar system. The nucleus represents the sun, and the electrons represent the planets orbiting around the nucleus. Unlike gravitational forces that keep planets in their .Ago 14, 2020 — The Bohr model of the hydrogen atom explains the connection between the quantization of photons and the quantized emission from atoms. Bohr described the hydrogen atom in terms of an electron moving in a circular orbit about a nucleus. He postulated that the electron was restricted to certain orbits characterized by discrete energies.Mar 5, 2024 — In the fluorine Bohr model, the nucleus holds 9 protons and 10 neutrons. Encircling this nucleus are two electron shells, carrying a total of 9 electrons. To draw the fluorine Bohr model, note the 9 protons, 10 neutrons, and 9 electrons. Start by sketching the nucleus, and then draw the two electron shells.In 1913, the physicist Niels Bohr introduced a model of the atom that contributed a greater understanding to its structure and quantum mechanics. Atoms are the basic units of chemical elements and were once believed to be the smallest indivisible structures of matter. The concept and terminology of the atom date as far back as ancient Greece, and different models were .
MediCard GO App is a new mobile application that provides easier access to your personal health needs. This app gives you the ability to request consultation or lab tests, securely speak to your doctor; schedule appointments; and access health coverage resources in an all-new, easy-to-use interface and navigation. .
bromine bohr model|3.6: Electron Arrangement